
#Charge of a proton free
A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.

Free neutrons do not directly ionize atoms, but they do indirectly cause ionizing radiation, so they can be a biological hazard, depending on dose. A free neutron spontaneously decays to a proton, an electron, and an antineutrino, with a mean lifetime of about 15 minutes. These events and findings led to the first self-sustaining nuclear reactor ( Chicago Pile-1, 1942) and the first nuclear weapon ( Trinity, 1945).ĭedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments. With the discovery of nuclear fission in 1938, it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, in a cascade known as a nuclear chain reaction. In the decade after the neutron was discovered by James Chadwick in 1932, neutrons were used to induce many different types of nuclear transmutations. The neutron is essential to the production of nuclear power. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutrons are produced copiously in nuclear fission and fusion. Neutrons are required for the stability of nuclei, with the exception of the single-proton hydrogen nucleus. With their positive charge, the protons within the nucleus are repelled by the long-range electromagnetic force, but the much stronger, but short-range, nuclear force binds the nucleons closely together. The properties of an atomic nucleus depend on both atomic and neutron numbers. Some elements occur in nature with only one stable isotope, such as fluorine Other elements occur with many stable isotopes, such as tin with ten stable isotopes, and some elements such as technetium have no stable isotope. For example, carbon, with atomic number 6, has an abundant isotope carbon-12 with 6 neutrons and a rare isotope carbon-13 with 7 neutrons. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus.Ītoms of a chemical element that differ only in neutron number are called isotopes. The number of neutrons is the neutron number.

The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus.
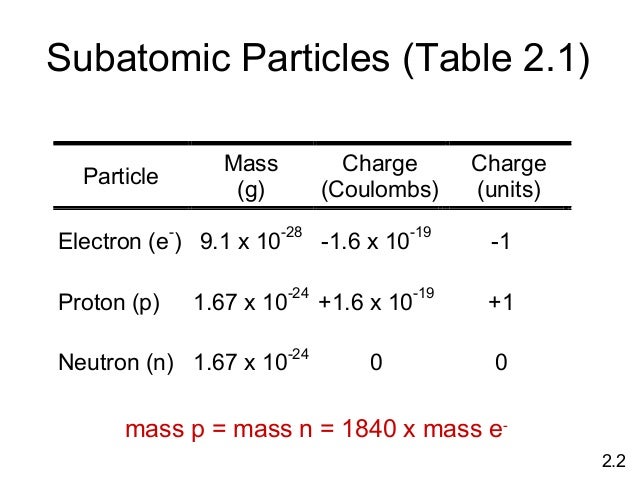
Their properties and interactions are described by nuclear physics. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.
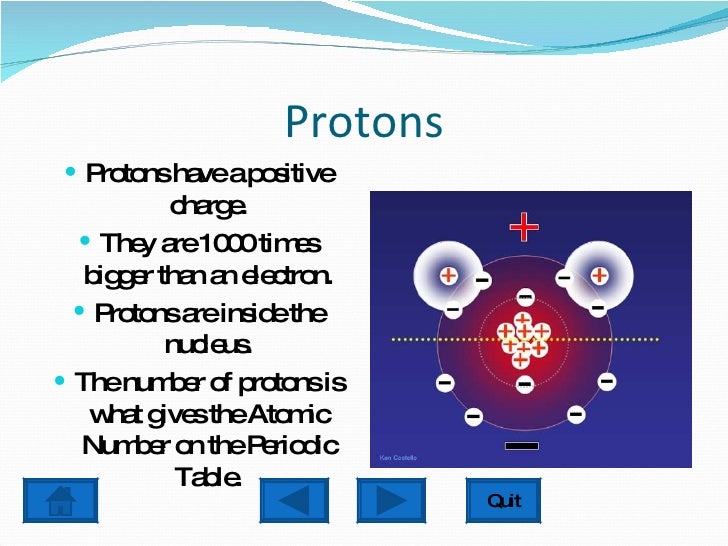
Protons and neutrons constitute the nuclei of atoms. , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. The neutron is a subatomic particle, symbol < 2.9 ×10 −26 e⋅cm (experimental upper limit) Forces between quarks are mediated by gluons. The color assignment of individual quarks is arbitrary, but all three colors must be present.


 0 kommentar(er)
0 kommentar(er)
